New York, USA, June 19, 2025 (GLOBE NEWSWIRE) — Dermatomyositis Market Set to Witness Significant Growth During the Study Period (2020–2034) Amid Rising Awareness and Advanced Treatments | DelveInsight
The dermatomyositis market is expected to witness steady growth over the next decade, driven by increasing awareness, improved diagnostic tools, and advancements in treatment options. The rising prevalence of autoimmune diseases globally, coupled with ongoing research into targeted biologics and immunotherapies, is further fueling market expansion. Additionally, regulatory support for orphan drugs and rare disease treatments is encouraging investment in dermatomyositis therapies. However, high treatment costs and diagnostic challenges may slightly restrain growth.
DelveInsight’s Dermatomyositis Market Insights report includes a comprehensive understanding of current treatment practices, emerging dermatomyositis drugs, market share of individual therapies, and current and forecasted dermatomyositis market size from 2020 to 2034, segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Key Takeaways from the Dermatomyositis Market Report
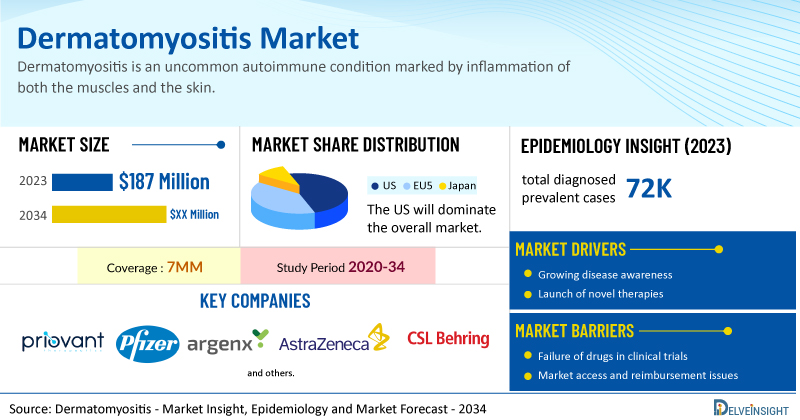
- According to DelveInsight’s analysis, the total dermatomyositis market size in the 7MM was estimated to be nearly USD 187 million in 2023, and it is expected to grow positively by 2034.
- The United States accounted for the highest Dermatomyositis market size approximately 62% of the total market size in 7MM in 2023.
- According to DelveInsight’s estimates, in 2023, there were nearly 72K total diagnosed prevalent cases of dermatomyositis in the 7MM, of which nearly 9% were juvenile and 91% were adult.
- Prominent companies, including Priovant Therapeutics, Pfizer, Argenx, AstraZeneca, CSL Behring, and others, are actively working on innovative dermatomyositis drugs. These novel dermatomyositis therapies are anticipated to enter the dermatomyositis market in the forecast period and are expected to change the market.
- Some of the key dermatomyositis treatments include Brepocitinib, Efgartigimod, Dazukibart (PF-06823859), SAPHNELO (anifrolumab), HIZENTRA (IgPro20), and others.
Discover which dermatomyositis medications are expected to grab the market share @ Dermatomyositis Market Report
Dermatomyositis Overview
Dermatomyositis is an uncommon autoimmune condition marked by inflammation of both the muscles and the skin. A defining symptom is weakness in the proximal muscles, those near the body’s core, such as the thighs and upper arms. Skin manifestations include a heliotrope rash, Gottron’s papules, and a rash that worsens with sun exposure.
Dermatomyositis diagnosis is based on clinical assessment, distinct physical signs, laboratory findings, and imaging studies. Core diagnostic criteria include proximal muscle weakness, characteristic skin rashes, elevated muscle enzymes, electromyography findings, and confirmation through muscle biopsy. Selecting the appropriate muscle for biopsy is critical for accurate diagnosis and to exclude other disorders. Conditions to consider in the differential dermatomyositis diagnosis include inclusion body myositis, myasthenia gravis, muscular dystrophies, motor neuron diseases, neuropathies, and inherited metabolic myopathies.
Dermatomyositis Epidemiology Segmentation
The dermatomyositis epidemiology section provides insights into the historical and current dermatomyositis patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The dermatomyositis market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
- Diagnosed Prevalent Cases of Dermatomyositis
- Age-specific Diagnosed Prevalent Cases of Dermatomyositis
- Gender-specific Diagnosed Prevalent Cases of Dermatomyositis
- Severity-specific Diagnosed Prevalent Cases of Dermatomyositis
- Chronicity-specific Diagnosed Prevalent Cases of Dermatomyositis
- Comorbidities-specific Diagnosed Prevalent Cases of Dermatomyositis
Download the report to understand which factors are driving dermatomyositis epidemiology trends @ Dermatomyositis Treatment Algorithm
Dermatomyositis Treatment Market
Currently, there is no definitive cure for dermatomyositis, but medications can help reduce inflammation and vasculitis, effectively lessen symptoms, and improve patients’ quality of life. The recent approval of OCTAGAM, along with various management guidelines, such as those from the British Society for Rheumatology and the Japanese Society of Rheumatology has significantly advanced treatment options for this condition. In addition, several off-label drugs, including corticosteroids, immunosuppressants, antimalarials, antibiotics, and topical ointments, are often used alone or in combination to manage symptoms.
Management of the skin manifestations of dermatomyositis involves general precautions, physiotherapy, and medical treatments. General care includes sun avoidance, wearing protective clothing, and applying sunscreen with SPF 30 or higher. Topical therapies typically involve corticosteroids and calcineurin inhibitors, while systemic treatments often use hydroxychloroquine and methotrexate. Systemic glucocorticoids tend to be effective for muscle symptoms but have limited impact on skin involvement.
Non-drug approaches include diet modifications, physical therapy, and adjunct treatments such as plasmapheresis, extracorporeal photochemotherapy, and total body irradiation for patients who do not respond to conventional therapy. Surgery is rarely necessary but may be used in cases of severe esophageal dysfunction (e.g., gastrostomy) or to remove calcinosis nodules. For dry eye symptoms, preventive strategies include environmental changes, patient education, dietary supplements (like fatty acids), ocular lubricants, and maintaining good eyelid hygiene.
Immunosuppressants and immunomodulators are often added alongside glucocorticoids to enhance treatment effectiveness and reduce the required steroid dose. Medications like azathioprine, methotrexate, mycophenolate mofetil, cyclophosphamide, tacrolimus, and cyclosporine may benefit patients who do not respond well to steroids alone, experience side effects, or have frequent relapses.
When corticosteroids are insufficient or long-term use presents risks, additional immunosuppressive drugs such as methotrexate, azathioprine, or mycophenolate mofetil are prescribed to further suppress the immune system, decreasing inflammation and muscle damage. Methotrexate, an antimetabolite, is commonly used as a first-line adjunct therapy for patients with inadequate response to oral corticosteroids. In adults, treatment starts at a low dose (7.5–10 mg weekly) and is gradually increased to about 25 mg per week. As the methotrexate dose increases, corticosteroid doses are reduced. Potential side effects of methotrexate include gastrointestinal, liver, and blood-related issues (such as neutropenia and thrombocytopenia). Folic acid supplementation is recommended to help minimize these adverse effects.
Learn more about the dermatomyositis treatment options @ Dermatomyositis Treatment Guidelines
Dermatomyositis Emerging Drugs and Companies
The dynamics of dermatomyositis market is anticipated to change in the coming years owing to the rising awareness of the disease, and development of vaccines across the world. Major key players involved in the development for the treatment of dermatomyositis are Brepocitinib by Priovant Therapeutics/Pfizer, Efgartigimod by Argenx, Dazukibart (PF-06823859) by Pfizer, SAPHNELO (anifrolumab) by AstraZeneca, HIZENTRA (IgPro20) by CSL Behring, and others.
Brepocitinib, an oral, once-daily therapy under development by Priovant Therapeutics, is a dual TYK2 and JAK1 inhibitor. It interferes with cytokine signaling pathways associated with autoimmune disorders, such as Type I and Type II interferons, IL-6, IL-12, and IL-23. By simultaneously targeting both TYK2 and JAK1, Brepocitinib aims to broadly suppress these immune pathways, which are key contributors to the development of dermatomyositis.
Efgartigimod, delivered subcutaneously, is a combination of efgartigimod alfa and recombinant human hyaluronidase PH20 (rHuPH20). It is a first-in-class investigational antibody fragment that targets the neonatal Fc receptor (FcRn). By binding to FcRn, efgartigimod disrupts its interaction with IgG, thereby reducing IgG recycling and promoting the breakdown of IgG and disease-causing autoantibodies, without impacting other immunoglobulins or albumin levels.
SAPHNELO (anifrolumab) is a fully human monoclonal antibody that binds to subunit 1 of the Type I interferon (IFN) receptor, blocking the activity of Type I IFNs. Additional Phase III studies are planned to evaluate its use in diseases where Type I IFN is central, including cutaneous lupus erythematosus, systemic sclerosis, and inflammatory myopathies such as dermatomyositis and polymyositis.
HIZENTRA is a 20% subcutaneous immunoglobulin solution derived from normal human plasma, containing at least 98% IgG. Over 90% of this IgG exists in the form of monomers and dimers, with aggregates kept very low (typically under 0.1%). The subclass distribution reflects that of natural plasma, with approximately 69% IgG1, 26% IgG2, 3% IgG3, and 2% IgG4. Proline (250 mmol/L) is included as a stabilizer, along with trace amounts of polysorbate 80 and sodium. HIZENTRA is approved for treating chronic inflammatory demyelinating polyneuropathy (CIDP) and primary immunodeficiency disorders.
Dazukibart (PF-06823859) is a humanized monoclonal IgG1κ antibody in development for dermatomyositis and polymyositis. It targets interferon beta-1 (IFNβ1), a molecule involved in regulating inflammatory responses and limiting immune cell infiltration into the central nervous system. By binding to IFNβ1, dazukibart inhibits signaling through the interferon alpha/beta receptor (IFNAR), thereby modulating the inflammatory pathways implicated in these diseases.
The anticipated launch of these emerging dermatomyositis therapies are poised to transform the dermatomyositis market landscape in the coming years. As these cutting-edge dermatomyositis therapies continue to mature and gain regulatory approval, they are expected to reshape the dermatomyositis market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about new treatment for dermatomyositis, visit @ Dermatomyositis Management
Dermatomyositis Market Dynamics
The dermatomyositis market dynamics are anticipated to change in the coming years. Increased awareness of dermatomyositis among healthcare professionals and the general public improves early diagnosis and treatment, potentially expanding market demand, while its status as a rare disease may qualify treatments for orphan drug incentives like tax breaks and market exclusivity, attracting pharmaceutical investment; additionally, growing governmental and private funding in rare disease research, alongside advancements in diagnostic technologies such as biomarkers and genetic testing, can accelerate treatment development and further broaden the market.
Furthermore, many potential therapies are being investigated for the treatment of dermatomyositis, and it is safe to predict that the treatment space will significantly impact the dermatomyositis market during the forecast period. Moreover, the anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate is expected to drive the growth of the dermatomyositis market in the 7MM.
However, several factors may impede the growth of the dermatomyositis market. As a rare disease with a relatively small patient population, dermatomyositis presents limited market potential that may deter large pharmaceutical companies, while the high research and development costs, serious associated complications like interstitial lung disease and cardiovascular disease, and stringent regulatory requirements with lengthy approval processes further increase barriers to entry and delay market availability.
Moreover, dermatomyositis treatment poses a significant economic burden and disrupts patients’ overall well-being and QOL. Furthermore, the dermatomyositis market growth may be offset by failures and discontinuation of emerging therapies, unaffordable pricing, market access and reimbursement issues, and a shortage of healthcare specialists. In addition, the undiagnosed, unreported cases and the unawareness about the disease may also impact the dermatomyositis market growth.
| Dermatomyositis Report Metrics | Details |
| Study Period | 2020–2034 |
| Dermatomyositis Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Dermatomyositis Market Size in 2024 | USD 187 Million |
| Key Dermatomyositis Companies | Priovant Therapeutics, Pfizer, Argenx, AstraZeneca, CSL Behring, and others |
| Key Dermatomyositis Therapies | Brepocitinib, Efgartigimod, Dazukibart (PF-06823859), SAPHNELO (anifrolumab), HIZENTRA (IgPro20), and others |
Scope of the Dermatomyositis Market Report
- Dermatomyositis Therapeutic Assessment: Dermatomyositis current marketed and emerging therapies
- Dermatomyositis Market Dynamics: Conjoint Analysis of Emerging Dermatomyositis Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s views, Analyst’s views, Dermatomyositis Market Access and Reimbursement
Discover more about dermatomyositis drugs in development @ Dermatomyositis Clinical Trials
Table of Contents
| 1. | Dermatomyositis Market Key Insights |
| 2. | Dermatomyositis Market Report Introduction |
| 3. | Dermatomyositis Market Overview at a Glance |
| 4. | Dermatomyositis Market Executive Summary |
| 5. | Disease Background and Overview |
| 6. | Dermatomyositis Treatment and Management |
| 7. | Dermatomyositis Epidemiology and Patient Population |
| 8. | Patient Journey |
| 9. | Dermatomyositis Marketed Drugs |
| 10. | Dermatomyositis Emerging Drugs |
| 11. | Seven Major Dermatomyositis Market Analysis |
| 12. | Dermatomyositis Market Outlook |
| 13. | Potential of Current and Emerging Therapies |
| 14. | KOL Views |
| 15. | Unmet Needs |
| 16. | SWOT Analysis |
| 17. | Appendix |
| 18. | DelveInsight Capabilities |
| 19. | Disclaimer |
| 20. | About DelveInsight |
Related Reports
Dermatomyositis Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Dermatomyositis companies, including Kezar Life Sciences, Argenx, Pfizer, CSL Behring, Viela Bio, PAEAN Biotechnology, Alexion Pharmaceuticals, among others.
Dermatomyositis Epidemiology Forecast
Dermatomyositis Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted dermatomyositis epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Polymyositis Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key polymyositis companies including Boehringer Ingelheim Pharmaceuticals, Mitsubishi Tanabe Pharma, Janssen Pharmaceutical, Kezar Life Sciences, Bristol-Myers Squibb, ONO Pharma, Horizon Therapeutics, Immunoforge, Restem, LLC., Eli Lilly and Company, among others.
Polymyositis Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key polymyositis companies, including Janssen Pharmaceutical, PAEAN Biotechnology, Kezar Life Sciences, Roche, Restem, LLC., Viela Bio, Bristol-Myers Squibb, ImmunoForge, among others.
Polymyositis Epidemiology Forecast
Polymyositis Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted polymyositis epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter



